
ELECTRICITY
What is electricity? First of all we must get acquainted with electricity itself. In an electrical dictionary written fifty years ago there is such a definition: Electricity. - The unknown thing, matter or force, or both, which is the cause of electric phenomena. Electricity is no longer an unknown thing. We know that electricity consists of particles, some of which may be made to move about and do work. We know the size of these particles, we know their mass, and we know how many of them must be moved to do any given amount of work. There is electricity in every gas, every liquid, and every solid substance which we know. All these substances are made up of different kinds of atoms in various combinations. Each atom consists of a central part, called the nucleus, around which, as in Fig. 1 whirl from one to 92 electrons. Electrons whirl around their nucleus as the Moon rotates around the Earth, and as the Earth and the other planets rotate around the Sun.
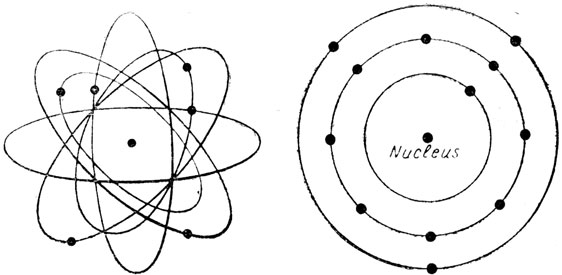
Fig. 1. Shelles in which electrons rotate.
Fig. 1. On the left is represented an atom of carbon with its central nucleus, around which rotate two electrons in an inner shell, and four more in an outer shell. Here is a simplified diagram of an aluminium atom having three shells in which are respectively two, eight and three electrons, making 13 electrons in all.
An atom is small. 250,000,000 atoms laid side by side in a row extend one inch. Yet the central nucleus of the inconceivably small atom occupies only one ten-thousandth of the diameter of the atom, and an electron is only about one-fifth the diameter of the nucleus. The only parts of the nucleus that interest us now are called protons, which are particles of positive electricity. We are much more interested in the electrons, which are particles of negative electricity. Our greater interest in the electrons arises from the fact that they can be separated from their atoms, and can be moved about and controlled. Electrons are the electricity with which we do things.
Positive and negative electricity, that is, protons and electrons have a strong attraction for each other. This attraction between the positive protons and the negative electrons holds the atom together.
ATOMS AND ELECTRONS
Now let's talk about a familiar substance - aluminium. In each atom of aluminium there are 13 negative electrons whirling around the positive nucleus. The attraction between these 13 particles of negative electricity and the positive protons in the nucleus is sufficient to hold the negative electrons in the atom under normal conditions. The positive force in the nucleus is equal to the negative forces of the 13 electrons as has been indicated in Fig. 2.
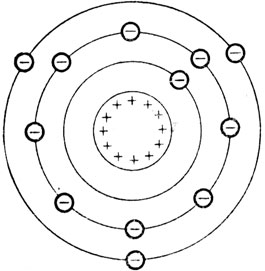
Fig. 2. The positive forces in the nucleus, represented by plus (+) signs, exactly balance the negative forces of all the electrons, represented by minus (-) signs.
In the atoms of aluminium and of many other substances one of the negative electrons farthest from the nucleus frequently breaks away from the atom to become a free electron. Ordinarily these free electrons almost immediately enter other atoms which have lost an electron because of the attraction between the positive electricity in the atoms and the negative electricity of the free electrons.
Assume that an aluminium atom has lost one of its negative electrons, as in Fig. 3. That free electron now is negative, it is a wandering particle of negative electricity. The nucleus of the atom still has as much positive electricity as ever, it still has enough positive electricity to attract and hold 13 negative electrons, but actually there are only 12 electrons remaining in the atom.
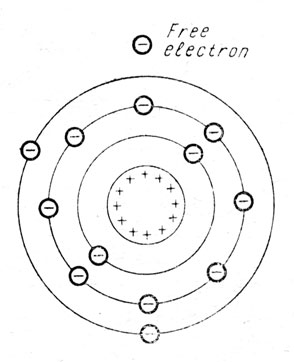
Fig. 3. Positive Atom. The aluminium atom has lost one electron from its outer shell, leaving the atom positive while the electron becomes a free particle of negative electricity.
This atom which has lost an electron contains more positive electricity than negative electricity. So we may call it a positive atom. This positive atom has strong attraction for any free negative electrons in its vicinity and that is the reason that remaining free electrons are continually re-entering the atoms.
aluminium алюминий
as much ... as ever столько же ... сколько всегда
attraction притяжение
break away (broke, broken) отделиться
carbon углерод
for each other зд. друг к другу
inconceivably непостижимо
inner внутренний
is only about one-fifth the diameter составляет всего около одной пятой диаметра
may be made to move about зд. можно заставить двигаться
minus (-) signs знаки «минус»
move about переходить (с места на место)
nucleus (nuclei - мн. ч.) ядро, центр
outer внешний
plus (+) signs знаки «плюс»
proton протон
rotate вращаться
in a row в ряд
shell оболочка
substance вещество
that is то есть
the only parts единственные части
in its vicinity поблизости
whirl around вертеться, кружиться
Experiment 1.
To determine the Specific Gravity of a liquid.
Apparatus:
The liquid (turpentine), water, density bottle.
Method:
The clean dry density bottle is weighed empty. It is then filled with turpentine and weighed again. It is emptied, washed thoroughly, filled with water and weighed again.
The weight of the bottle is subtracted from weighings 2 and 3.
| Sp. Grav. of Turps = | Weight of turps, which fills bottle | |
| Weight of same vol. of water |
Results:
Weight of empty bottle =………….
Weight of bottle full of turps =………..
Weight of bottle full of water = ………….
Wt. of bottle full of turps Wt. of bottle full of water
| Sp. Grav. of turps = | Wt. bottle full of turps | |
| Wt. bottle full of water |
Precautions:
1) Care is taken to see that the bottle is full each time, including the hole in the stopper.
2) The bottle is dried thoroughly outside before each weighing.
care is taken to see that принимаются меры предосторожности
density bottle сосуд для измерения плотности
Sp. Grav = Specific Gravity удельный вес
stopper пробка
turpentine (turps) скипидар
vol. = volume объем
wt. = weight вес
Experiment 2.
To verify the Principle of Archimedes.
Apparatus:
Bucket and cylinder, water and pipette, balance and weights, bridge, beaker. Fig. 1.
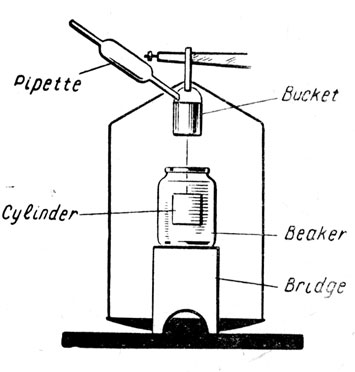
Fig. 1.
Method:
The bucket with the cylinder below it is hung from the balance arm and weights are put in the other pan to counterbalance it.
The bridge is placed over the pan and a beaker of water arranged on it so that the cylinder is in the water but the bucket above it.
It is now found that the weights in the pan are too heavy.
Water is added to the bucket from the pipette until the two sides balance. This occurs when the bucket is exactly full of water, i. e. when water equal to the volume of the cylinder has been added.
This shows that the apparent loss in weight of the cylinder is equal to the weight of the water displaced.
balance arm коромысло весов
balance and weights весы и гири
beaker мензурка
bridge мостик, подставка
counterbalance уравновешивать
it is now found зд, теперь находим
pan чашка весов
pipette пипетка
verify подтверждать
Experiment 3.
To determine the Specific Gravity of a solid denser than water by the Principle of Archimedes. Apparatus:
Solid (glass stopper), thread, bridge, beaker of water, balance and weights. Fig. 2.
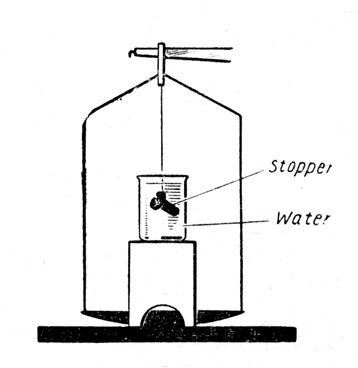
Fig. 2.
Method:
The glass stopper is hung from the hook of the balance arm and weighed.
The bridge and beaker are then placed in position so that the stopper hangs in the water.
The stopper is weighed again and care is taken that the stopper is completely immersed.
The apparent loss in weight of the stopper (i. e. the weight of an equal volume of water) is found.
| Sp. Grav. of = | Weight of stopper in air | |
| Apparent loss in weight of stopper |
Results:
Wt. of stopper in air = ……..
Wt. of stopper in water = ……
Apparent loss in weigh = ..........
| Sp. Grav. of glass = | Wtю of stopper in air | |
| Apparent loss in weight |
the apparent loss видимая потеря
is completely immersed зд. полностью погружена
Experiment 4.
To compare the densities of two liquids or to determine the density of one
a) with a U-tube,
b) with Hare's apparatus.
Apparatus:
a) U-tube, mercury, 2 liquids or one liquid and water, pipette, metre-rule.
Hare's apparatus, 2 beakers, 2 liquids or one liquid and water, metre-rule. Fig. 3.
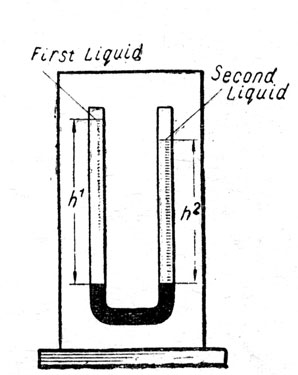
Fig. 3.
b) A little mercury is poured into the U-tube to prevent the liquids mixing.
The other liquid (or water) is put into the second arm until the two mercury columns are level. Final adjustment is made with the pipette.
The lengths of the two columns of liquid above the mercury are measured.
Results:
Let d1 and d2 gm. (cc.) be the densities of the liquids.
Let h1 and h2 cm. be the heights of their respective columns.
Then, in each case:
h1d1=h2d2,
or
| d1 | = | h1 | . |
| d2 | h2 |
If the one liquid is water (where d1 - 1 gm./cc,), then d2 can be calculated.
column колонка
half-full наполненная наполовину
with Hare's apparatus аппаратура Харе
are level зд. на одном уровне
metre-rule метрическая линейка
prevent the liquids mixing для того, чтобы помешать перемешиванию жидкостей
a U-tube У-образиая трубка
Experiment 5.
То construct a simple Hydrometer.
Apparatus:
Narrow test-tube with flat bottom, lead shot, stiff lined paper for scale.
Method:
The test-tube is weighed with lead shot to make it float upright. The strip of paper is placed inside to act as a scale.
The tube is floated in water and the depth submerged is marked l1.
It is then floated in a second liquid and the depth submerged marked l2.
Weight of hydrometer = weight of liquid displaced = l × a × d
(where l - depth submerged, a = area of cross section, d = density of liquid).
Hence l1 × a × d1= l2 × a × d2.
Whence
| d1 | = | l1 | . |
| d2 | l2 |
act as a scale зд. для того, чтобы действовать как весы
area of cross section площадь поперечного сечения
the depth submerged зд. глубина погружения
hydrometer гидрометр
make it float upright зд. для того, чтобы она плавала прямо
for scale для масштаба
2nd liquid - second liquid вторая жидкость
test-tube пробирка
Experiment 6.
То verify Boyle's Law:
a) with a J-tube,
b) with Boyle's Law Apparatus.
Apparatus:
a) J-tube, mercury, small funnel, metre-rule.
b) Boyle's Law Apparatus. Fig. 4.
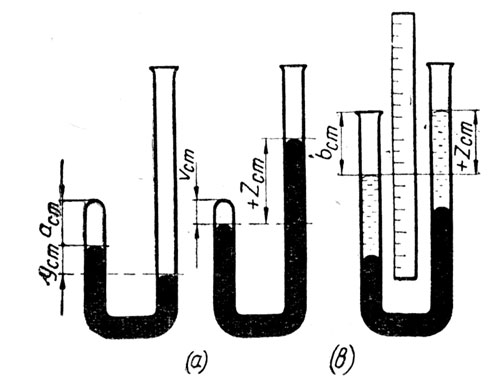
Fig. 4.
Method:
a) Some mercury is poured into the open side of the J-tube and the tube is tilted to allow some of the trapped air to escape, until the mercury on the closed side is higher than that on the open side. The difference in level of the two sides is recorded and the length of the trapped air column is measured.
Mercury is added to the open side and the difference in level and length of the air column are again recorded.
This is repeated several times and the results are tabulated.
The height of the barometer is taken and the pressure of the enclosed air is found for each reading.
b) The open side of the apparatus is adjusted until both mercury columns are level. The length (or volume) of the trapped air column is measured.
The open side is lowered and several readings are taken with the open column below the closed one.
Then it is raised and several more readings taken with the open column above the closed one.
The height of the barometer is taken and the air pressures found as before.
Results:
| Length of air column | Differ. in level of merc. columns | - | Pressure of air enclosed | (PV) |
| a сm. | (open) - y cm. (below) (closed) | A - y cm. | 1/a | a(A - y) |
| b сm. | (open) + z cm. (above) (closed) | A + z cm. | 1/b | b(A + z) |
Height of Barometer = A cm. of mercury.
| 1 | ||
| V |
is plotted against P and a straight line graph verifies the law.
(The product PV should be a constant.)
on the closed side с закрытой стороны
constant постоянный
differ. = difference различие, разность
funnel воронка
a J-tube пробирка (такой формы)
mere. - mercury ртуть
with the open column below the closed one зд. причем открытый столбик ниже закрытого
plot наносить (на график)
several more readings taken берется еще несколько показаний
Experiment 7.
То determine the Specific Gravity of a solid less dense than water by the Principle of Archimedes. Apparatus:
Solid (cork), thread, metal sinker, bridge, beaker of water, balance and weights. Fig. 5.
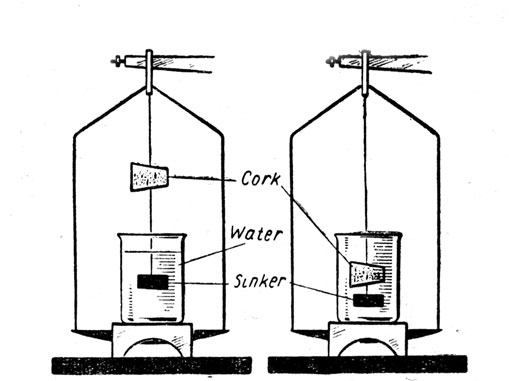
Fig. 5.
Method:
The cork is weighed in air (W1).
It is then hung from the hook of the balance arm and the sinker is suspended from it by a piece of thread so that the cork is in air and the sinker is in a beaker of water placed on the bridge. The combined weight is found (W2).
The cork is then fastened to the sinker and the two are weighed suspended in the beaker of water (W3).
The difference between W2 and W3 is the apparent loss in weight of the cork (i. e. the weight of the water displaced).
Hence the Specific Gravity of cork can be found.
Results:
Weight of cork in air = ....
Weight of cork in air and sinker in water = ....
Weight of cork and sinker in water = ....
Apparent loss in weight of cork = ....
| Specific Gravity of corK = | Wt. of cork | . |
| Apparent loss in wt. |
combined weight общий вес
cork пробка
metal sinker металлический груз, грузило
suspended in взвешенный в
Experiment 8.
To determine the density of air.
Apparatus:
Round-bottomed flask fitted with rubber bung, short piece of glass tubing, piece of thick-walled rubber tubing and screw-clip. Filter pump, balance and weights, bowl large enough to contain the flask. Fig. 6.
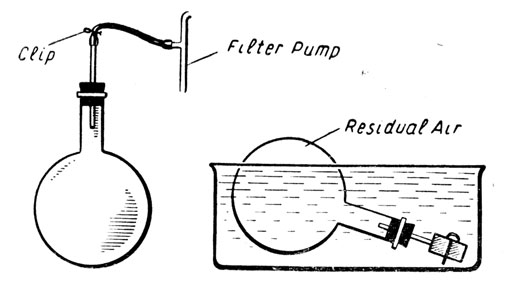
Fig. 6.
Method:
The flask is weighed full of air at atmospheric pressure with the clip open.
It is then attached to the filter pump for two or three minutes. The clip is screwed up tight and the weight of air withdrawn is found by re-weighing.
The clip is unscrewed under water, and when the flask has filled up and the residual air is once more at atmospheric pressure (the surface of the water in the flask must be level with the surface of the water in the bowl), the clip is closed and the volume of water in the flask is found by weighing. This equals the volume of the air withdrawn.
| Density of air = | Weight of air withdrawn | . |
| Volume of air withdrawn |
Results:
Weight of flask full of air = ....
Weight of flask after withdrawal = ....
Weight of flask with water = ....
Weight of air withdrawn = ....
Weight of water in flask =….
Volume of water in flask =….
| Density of air = | Weight of air withdrawn | . |
| Weight of same volume of air water |
of air withdrawn зд. удаленного воздуха
bowlтигель, резервуар
fitted зд. снабженная
flask колба
glass tubing стеклянные трубы
large enough to contain достаточно большая, чтобы вместить
residual air оставшийся (остаточный) воздух
re-weight повторно взвешивать
round bottomed flask колба с круглым дном
rubber bung резиновая (большая) пробка
screw-clip завинчивающийся зажим
is screwed up tight туго завинчивается
unscrew отвинчивать
|
ПОИСК:
|
© GENLING.RU, 2001-2021
При использовании материалов сайта активная ссылка обязательна:
http://genling.ru/ 'Общее языкознание'
При использовании материалов сайта активная ссылка обязательна:
http://genling.ru/ 'Общее языкознание'